Stereocontrolled synthesis and investigation of the biosynthetic transformations of 16(S),17(S)-epoxy-PDn-3 DPA†
Abstract
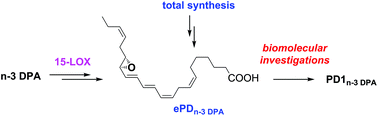
PD1n-3 DPA is a specialized pro-resolving lipid mediator that displays potent anti-inflammatory properties and pro-resolving bioactivities. Such naturally occurring compounds are of current interest in biomolecular chemistry and drug discovery. To investigate the involvement of an epoxide intermediate in the biosynthesis of PD1n-3 DPA from n-3 docosapentaenoic acid, the epoxy acid 16(S),17(S)-epoxy-PDn-3 DPA, herein named ePDn-3 DPA, was prepared by stereoselective total synthesis. The synthetic material of ePDn-3 DPA allowed investigations of its role in the biosynthesis of PD1n-3 DPA. The obtained results establish that the biosynthesis of PD1n-3 DPA in neutrophils occurs with ePDn-3 DPA as the intermediate, and that 15-LOX produces ePDn-3 DPA from n-3 docosapentaenoic acid. Furthermore, support for the involvement of a hydrolytic enzyme in the biosynthetic conversion of ePDn-3 DPA to PD1n-3 DPA was found. In addition, ePDn-3 DPA was found to regulate the formation of the potent neutrophil chemoattractant LTB4 with equal potencies to that obtained with PD1n-3 DPA.


 Please wait while we load your content...
Please wait while we load your content...