Palladium-catalyzed tandem reaction of 2-chloroquinoline-3-carbaldehydes and isocyanides†
Abstract
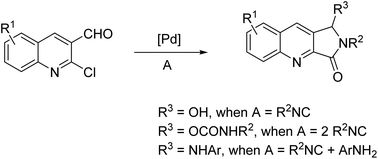
A facile domino reaction of 2-chloroquinoline-3-carbaldehydes in one and two equivalents of isocyanide has been investigated. Three-component reactions of 2-chloroquinoline-3-carbaldehydes, isocyanides and amines are also described. In this Pd-catalyzed reaction under controlled conditions, three novel types of quinoline derivatives were formed via amidation, lactamization or carbamate formation along with the formation of C–C, C–N, and C–O bonds in a one-pot procedure.


 Please wait while we load your content...
Please wait while we load your content...