In vitro biocatalytic pathway design: orthogonal network for the quantitative and stereospecific amination of alcohols†
Abstract
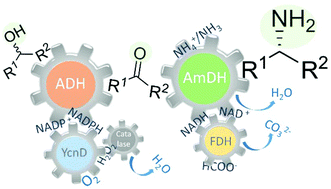
The direct and efficient conversion of alcohols into amines is a pivotal transformation in chemistry. Here, we present an artificial, oxidation–reduction, biocatalytic network that employs five enzymes (alcohol dehydrogenase, NADP-oxidase, catalase, amine dehydrogenase and formate dehydrogenase) in two concurrent and orthogonal cycles. The NADP-dependent oxidative cycle converts a diverse range of aromatic and aliphatic alcohol substrates to the carbonyl compound intermediates, whereas the NAD-dependent reductive aminating cycle generates the related amine products with >99% enantiomeric excess (R) and up to >99% conversion. The elevated conversions stem from the favorable thermodynamic equilibrium (K′eq = 1.88 × 1042 and 1.48 × 1041 for the amination of primary and secondary alcohols, respectively). This biocatalytic network possesses elevated atom efficiency, since the reaction buffer (ammonium formate) is both the aminating agent and the source of reducing equivalents. Additionally, only dioxygen is needed, whereas water and carbonate are the by-products. For the oxidative step, we have employed three variants of the NADP-dependent alcohol dehydrogenase from Thermoanaerobacter ethanolicus and we have elucidated the origin of the stereoselective properties of these variants with the aid of in silico computational models.


 Please wait while we load your content...
Please wait while we load your content...