Tandem radical cyclization to construct poly-brominated 2-oxindoles†
Abstract
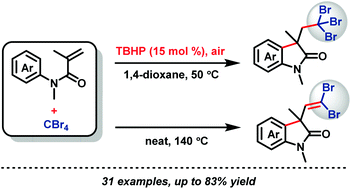
Catalytic amounts of TBHP (15 mol%) promoted tribromomethylation of activated alkenes has been developed. This method provided a metal-free aerobic way to construct tribromomethylated 2-oxindoles from the reaction of readily available N-arylacrylamides with CBr4via a proposed tandem radical cyclization process. Air is used as an efficient terminal oxidant in this transformation. The formation of 1,1-dibromoolefin derivatives was also realized at higher temperature under neat conditions.


 Please wait while we load your content...
Please wait while we load your content...