Accessing 2-substituted piperidine iminosugars by organometallic addition/intramolecular reductive amination: aldehyde vs. nitrone route†
Abstract
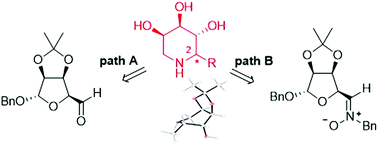
A dual synthetic strategy to afford 2-substituted trihydroxypiperidines is disclosed. The procedure involved Grignard addition either to a carbohydrate-derived aldehyde or to a nitrone derived thereof, and took advantage of an efficient ring-closure reductive amination strategy in the final cyclization step. An opposite diastereofacial preference was demonstrated in the nucleophilic attack to the two electrophiles, which would finally produce the same piperidine diastereoisomer as the major product. However, use of a suitable Lewis acid in the Grignard addition to the nitrone allowed reversing the selectivity, giving access to 2-substituted piperidines with the opposite configuration at C-2.


 Please wait while we load your content...
Please wait while we load your content...