Intramolecular hydrogen bonding in conformationally semi-rigid α-acylmethane derivatives: a theoretical NMR study†
Abstract
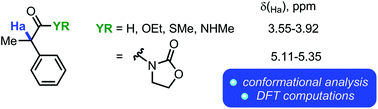
Conformational mobility is a core property of organic compounds, and conformational analysis has become a pervasive tool for synthetic design. In this work, we present experimental and computational (employing Density Functional Theory) evidence for unusual intramolecular hydrogen bonding interactions in a series of α-acylmethane derivatives, as well as a discussion of the consequences thereof for their NMR spectroscopic properties.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...