Enantioselective electrophilic cyanation of β-keto amides catalysed by a cinchona organocatalyst†
Abstract
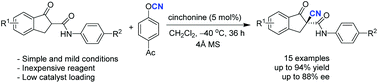
An operationally simple protocol for the enantioselective electrophilic α-cyanation of β-keto amides catalyzed by cinchona-derived catalysts has been demonstrated. The resulting products could be obtained with good to high enantioselectivities (up to 88% ee) and with excellent yields (up to 94%) by employing the mild active 4-acetylphenyl cyanate as the cationic cyano source in the catalytic asymmetric α-cyanation reaction.


 Please wait while we load your content...
Please wait while we load your content...