Synthesis of 4-benzylpyridines via Pd-catalyzed CH3-arylation of 4-picoline†
Abstract
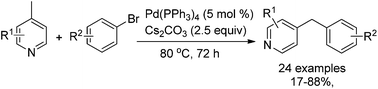
A highly efficient synthesis of 4-benzylpyridines was developed via Pd-catalyzed C(sp3)–H arylation between 4-picoline and aryl halides. It was found that the best yields were achieved with a simple Pd(PPh3)4 catalyst and Cs2CO3 as the base. Compared with the known methods, our reaction does not require the use of a strong organometallic reagent as the base.


 Please wait while we load your content...
Please wait while we load your content...