tert-Butyl nitrite (TBN) as the N atom source for the synthesis of substituted cinnolines with 2-vinylanilines and a relevant mechanism was studied†
Abstract
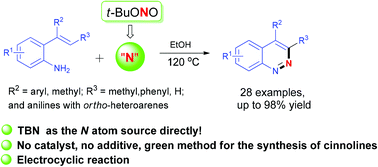
A green method to synthesize cinnolines by 6π electrocyclic reaction with alkenyl amines and TBN has been developed. TBN plays a dual role both as a nitrogen atom source and an oxidant in this procedure. Relevant mechanism experiments reveal that the reaction proceeds through electrocyclic reaction and with diazo hydroxide as a key intermediate.


 Please wait while we load your content...
Please wait while we load your content...