On the discovery of new potent human farnesyltransferase inhibitors: emerging pyroglutamic derivatives†
Abstract
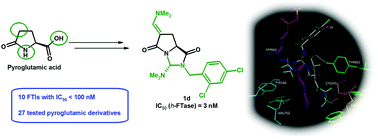
In the current context of lack of emergence of innovative human farnesyltransferase inhibitors families, and given all new therapeutic perspectives that open up for such molecules in rare diseases (e.g. Hutchinson–Gilford progeria syndrome), and in delta hepatitis, cardiovascular or neuroinflammatory diseases, we have just discovered a new series of powerful inhibitors. These molecules are pyroglutamic acid derivatives, and were evaluated on human farnesyltransferase in vitro then modeled in silico on the active site of the protein. Three main points of the pyroglutamic acid cycle have undergone chemical modulations pyroglutamides in position 5 (compounds 7a–h), constrained bicyclic analogues of pyrroloimidazoledione type (compounds 1a–h), modulation of the position 3 (compounds 2–5 and 8), and allowed the first SAR in the field. Five derivatives in the current work have IC50 values in the small nanomolar range (2–5 nM). These new lead compounds open the way for the next generation of farnesyltransferase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...