Low pH-triggering changes in peptide secondary structures†
Abstract
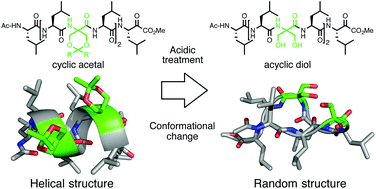
We developed a novel methodology using cyclic α,α-disubstituted α-amino acids (dAAs) with an acetal-side chain to control peptide secondary structures. The introduction of cyclic dAAs into peptides contributed to the stabilization of peptide secondary structures as a helix, while an acidic treatment of peptides resulted in a marked conformational change.


 Please wait while we load your content...
Please wait while we load your content...