Copper-catalyzed decarboxylative methylthiolation of aromatic carboxylate salts with DMSO†
Abstract
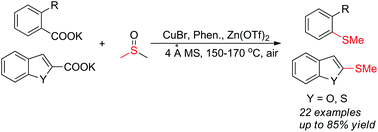
A novel copper-catalyzed decarboxylative methylthiolation of arenecarboxylate salts has been realized using DMSO as the methylthiolation source. Various potassium aryl carboxylates underwent decarboxylative methylthiolation under air to furnish the corresponding aryl methyl thioethers in moderate to excellent yields. The reaction tolerated a wide variety of functional groups. Notably, the synthesis of ethylthioethers was also successfully achieved directly from diethyl sulfoxide under similar reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...