Deuteration and tautomeric reactivity of the 1-methyl functionality of free-base dipyrrins†
Abstract
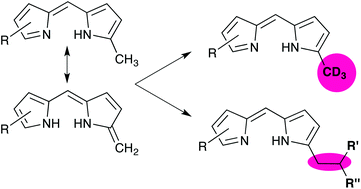
Regioselective reactivity of the 1-methyl group of free-base dipyrrins is explored, including discussion of tautomerism to provide exocyclic alkenyl reactivity. Deuterium is installed so as to generate dipyrrins substituted with deuterated methyl groups. Furthermore, the 1-methyl group reacts to become involved in C–C bonds involving only sp3-hybridised carbon atoms. The isolation of an elusive framework featuring a dipyrrin substituted with a pyrrole in a non-vinylogous fashion is also reported. The use of asymmetric dipyrrins featuring an electron-withdrawing group on one of the pyrrolic units results in regioselective reaction of the alpha-methyl group distal to the electron-withdrawing group.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...