Photoredox-catalyzed procedure for carbamoyl radical generation: 3,4-dihydroquinolin-2-one and quinolin-2-one synthesis†
Abstract
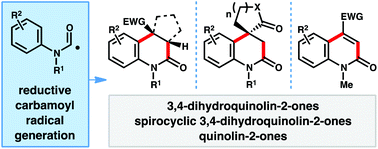
A reductive approach for carbamoyl radical generation from N-hydroxyphthalimido oxamides under photoredox catalysis is outlined. This strategy was applied to the synthesis of 3,4-dihydroquinolin-2-ones via the intermolecular addition/cyclization of carbamoyl radicals with electron deficient olefins in a mild, redox-neutral manner. Under a general set of reaction conditions, diversely substituted 3,4-dihydroquinolin-2-ones, including spirocyclic systems can be prepared. By using chlorine-substituted olefins, aromatic quinolin-2-ones can also be accessed.


 Please wait while we load your content...
Please wait while we load your content...