Rhodium-catalyzed redox-neutral coupling of phenidones with alkynes†
Abstract
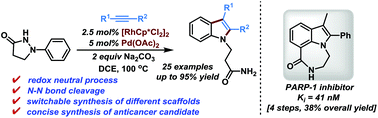
A switchable synthesis of N-substituted indole derivatives from phenidones via rhodium-catalyzed redox-neutral C–H activation has been achieved. In this protocol, we firstly disclosed that the reactivity of Rh(III) catalysis could be enhanced through employing palladium acetate as an additive. Some representative features include external oxidant-free, applicable to terminal alkynes, short reaction time and operational simplicity. The utility of this method is further showcased by the economical synthesis of potent anticancer PARP-1 inhibitors.


 Please wait while we load your content...
Please wait while we load your content...