Unified total synthesis of (+)-chinensiolide B and (+)-8-epigrosheimin†
Abstract
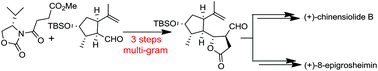
An expedient synthetic approach has been developed for the unified total synthesis of (+)-chinensiolide B and (+)-8-epigrosheimin. The point of divergence was provided by the lactone aldehyde 6, in which four contiguous stereocenters were achieved by a stereocontrolled Evans syn-aldol reaction of a R-carvone derived enantiopure aldehyde and chiral N-succinyl-oxazolidinone. The lactone aldehyde 6 was synthesized in multigram quantity in three steps. Highly optimized chemo- and stereoselective reactions and functional group interconversion enabled us to assemble (+)-chinensiolide B and (+)-8-epigrosheimin from 6.


 Please wait while we load your content...
Please wait while we load your content...