Mechanism of Pd-catalyzed acylation/alkenylation of aryl iodide: a DFT study†
Abstract
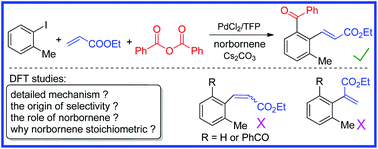
The Pd-catalyzed cross-coupling of aryl iodide, benzoic anhydride and ethyl acrylate provided a useful alternative to synthesize alkenylated aryl ketones with good selectivity and functional-group tolerance. In this manuscript, density functional theory (DFT) calculations were performed to address the detailed reaction mechanism. Computational results support the experimentally proposed Pd(0)–Pd(II)–Pd(IV) catalytic cycle and further clarify that the rate-determining step is the oxidative addition of benzoic anhydride, the regioselectivity-determining step is the migratory insertion of ethyl acrylate and the following β-H elimination determines the stereoselectivity. The regioselectivity can be attributed to the steric and electronic effect of ethyl acrylate and the stereoselectivity can be explained by the steric repulsion between the toluene moiety and the CO2Et moiety. Furthermore, we found that norbornene not only acts as the removable scaffold in Catellani–Lautens-type reactions, but also plays the role of suppressing the competitive migratory insertion of ethyl acetate. Norbornene insertion and ethyl acetate insertion are found to have close free energy barriers, which shed light on the origin of using stoichiometric amounts of norbornene.


 Please wait while we load your content...
Please wait while we load your content...