Synthesis of functionalized alkyl substituted benzoquinones by Rh-catalyzed additions of boronic acids†
Abstract
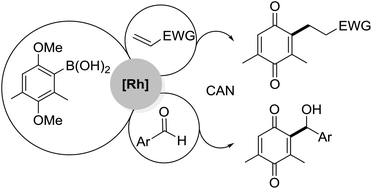
A general synthetic route to γ-oxo alkyl or α-hydroxy benzyl 2-substituted benzoquinones has been developed through a one-pot Rh-catalyzed C–C bond formation/oxidative demethylation sequence from 2,5-dimethoxy aryl boronic acids and several electron deficient alkenes or aldehydes. The process allows rapid access to functionalized benzoquinones under very mild conditions and good yields. We disclose the first example of a Rh-catalyzed 1,4-addition reaction of benzoquinonyl boronic acid to methyl vinyl ketone and other conjugate acceptors, which allows the direct synthesis of 2-(γ-functionalized alkyl) substituted benzoquinones.


 Please wait while we load your content...
Please wait while we load your content...