Divergent syntheses of iodinated isobenzofuranones and isochromenones by iodolactonization of 2-alkynylbenzoic acids in ionic liquids†
Abstract
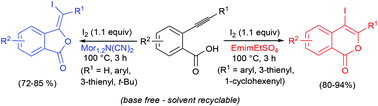
The regiochemical outcome of the iodolactonization of 2-alkynylbenzoic acids, carried out at 100 °C in ionic liquids (ILs) as unconventional solvents and with molecular iodine as the iodine source, in the absence of external bases, was found to be strongly dependent on the nature of the IL medium. In particular, while the use of N-ethyl-N-methylmorpholinium dicyanamide (Mor1,2N(CN)2) promoted the stereoselective formation of (E)-3-(iodomethylene)isobenzofuran-1(3H)-ones, through an anti-5-exo-dig cyclization route, the use of 1-ethyl-3-methylimidazolium ethyl sulfate (EmimEtSO4) tended to favor the 6-endo-dig cyclization mode, with preferential or selective formation of 4-iodo-1H-isochromen-1-ones. In any case, the IL solvent could be easily recycled after extraction of the product from the reaction mixture with diethyl ether. DFT calculations have been carried out to clarify the role of the IL's nature in favoring either the anti-5-exo-dig cyclization route or the 6-endo-dig mode. In the case of iodocyclization of 2-ethynylbenzoic acid, only the 5-exo-dig mode was observed in both EmimEtSO4 and Mor1,2N(CN)2 solvents. The structures of two representative products have been confirmed by X-ray diffraction analysis.


 Please wait while we load your content...
Please wait while we load your content...