Formal total synthesis of selaginpulvilin D†
Abstract
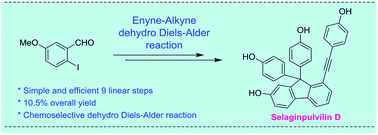
An efficient and mild synthetic strategy for the total synthesis of selaginpulvilin D has been reported. A highly chemoselective enyne–alkyne dehydro Diels–Alder reaction has been employed for the construction of the tricyclic fluorene framework present in the natural product selaginpulvilin D. An improved overall yield (10.5%) has been achieved for selaginpulvilin D, starting from commercially available m-anisaldehyde in 9 linear, operationally simple synthetic transformations.


 Please wait while we load your content...
Please wait while we load your content...