Prodigiosenes conjugated to tamoxifen and estradiol†
Abstract
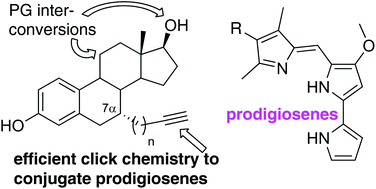
We report the synthesis of the first click-appended prodigiosene conjugates. Four prodigiosene conjugates of estradiol functionalised at the 7α-position were prepared, as were three prodigiosene conjugates of tamoxifen. The coupling between a prodigiosene and an 11-hydroxy estradiol derivative via an ether linkage was investigated, as was the 11- and 7-functionalisation of the estradiol core. The robustness of estradiol protecting groups was severely challenged by reactions typically used to equip such frameworks for 11- and 7-functionalisation. Specifically, and important to synthesis involving estradiol, TBS, TMS and THP are not useful protecting groups for the functionalisation of this core. When the chemical features of the therapeutic agent limit the choice of protecting group (in this case, prodigiosenes bearing aryl, NH, alkenyl and ester groups), click chemistry becomes an attractive synthetic strategy. The anti-cancer activity of the seven click prodigiosene conjugates was evaluated.


 Please wait while we load your content...
Please wait while we load your content...