Nitrile-assisted oxidation over oxidative-annulation: Pd-catalyzed α,β-dehydrogenation of α-cinnamyl β-keto nitriles†
Abstract
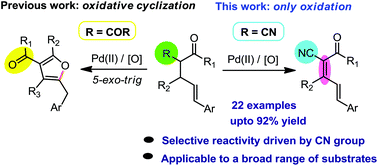
A palladium-catalyzed oxidation reaction is disclosed where the nitrile functionality on the substrate simply changes the course of the reaction. Our previous finding showed that using the Pd(II)-catalyst in the presence of benzoquinone as an oxidant, 2-cinnamyl-1,3-dicarbonyls provides functionalized furans via oxidative cyclization. When a nitrile group is replaced with one of the carbonyl functionalities of the same substrate, the oxidative cyclization was completely suppressed; instead, the oxidation at the α,β-position occurred to provide α,β,γ,δ-diene containing β-keto nitriles.
- This article is part of the themed collection: Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...