Diastereoselective synthesis and biological evaluation of enantiomerically pure tricyclic indolines†
Abstract
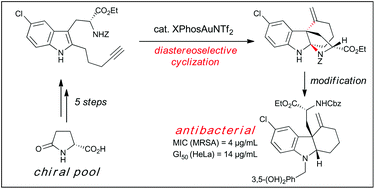
Tricyclic indolines are common in both natural products and synthetic chemical probes. In this study we demonstrated that enantiomerically pure tricyclic indolines can be prepared from an inexpensive commercially available chiral starting material, pyroglutamic acid. The synthesis features a highly diastereoselective gold-catalyzed cyclization of alkyne-tethered indoles and subsequent diastereoselective reductive ring-opening reaction. Using this approach, we synthesized analogs of our previously discovered tricyclic indoline probes that possess antibacterial and resistance-modifying activity. The biological activity against methicillin-resistant Staphylococcus aureus (MRSA) of these analogues was evaluated and reported. The synthetic approach reported may be leveraged in the future to prepare diastereopure chemical probes for the determination of biological targets for drug discovery.


 Please wait while we load your content...
Please wait while we load your content...