Copper-catalyzed tandem aryl–halogen hydroxylation and CH2Cl2-based N,O-acetalization toward the synthesis of 2,3-dihydrobenzoxazinones†
Abstract
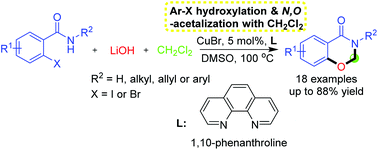
The concise synthesis of 2,3-dihydro-4H-benzo[e][1,3]oxazin-4-ones has been accomplished by copper-catalyzed tandem reactions of o-halobenzamides, LiOH and dichloromethane. The aryl–halogen bond hydroxylation and subsequent N,O-acetalization on CH2Cl2 are enabled under catalytic conditions which allows the generation of C(sp2)–O, C(sp3)–O and C(sp3)–N bonds to give the target products.


 Please wait while we load your content...
Please wait while we load your content...