Expanding the substrate scope of phenylalanine ammonia-lyase from Petroselinum crispum towards styrylalanines†
Abstract
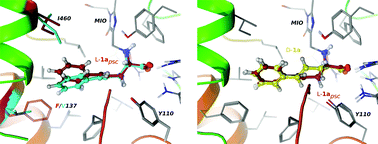
This study focuses on the expansion of the substrate scope of phenylalanine ammonia-lyase from Petroselinum crispum (PcPAL) towards the L-enantiomers of racemic styrylalanines rac-1a–d – which are less studied and synthetically challenging unnatural amino acids – by reshaping the aromatic binding pocket of the active site of PcPAL by point mutations. Ammonia elimination from L-styrylalanine (L-1a) catalyzed by non-mutated PcPAL (wt-PcPAL) took place with a 777-fold lower kcat/KM value than the deamination of the natural substrate, L-Phe. Computer modeling of the reactions catalyzed by wt-PcPAL indicated an unproductive and two major catalytically active conformations and detrimental interactions between the aromatic moiety of L-styrylalanine, L-1a, and the phenyl ring of the residue F137 in the aromatic binding region of the active site. Replacing the residue F137 by smaller hydrophobic residues resulted in a small mutant library (F137X-PcPAL, X being V, A, and G), from which F137V-PcPAL could transform L-styrylalanine with comparable activity to that of the wt-PcPAL with L-Phe. Furthermore, F137V-PcPAL showed superior catalytic efficiency in the ammonia elimination reaction of several racemic styrylalanine derivatives (rac-1a–d) providing access to D-1a–d by kinetic resolution, even though the D-enantiomers proved to be reversible inhibitors. The enhanced catalytic efficiency of F137V-PcPAL towards racemic styrylalanines rac-1a–d could be rationalized by molecular modeling, indicating the more relaxed enzyme–substrate complexes and the promotion of conformations with higher catalytic activities as the main reasons. Unfortunately, ammonia addition onto the corresponding styrylacrylates 2a–d failed with both wt-PcPAL and F137V-PcPAL. The low equilibrium constant of the ammonia addition, the poor ligand binding affinities of 2a–d, and the non-productive binding states of the unsaturated ligands 2a–d within the active sites of either wt-PcPAL or F137V-PcPAL – as indicated by molecular modeling – might be responsible for the inactivity of the PcPAL variants in the reverse reaction. Modeling predicted that the F137V mutation is beneficial for the KRs of 4-fluoro-, 4-cyano- and 4-bromostyrylalanines, but non-effective for the KR process of 4-trifluoromethylstyrylalanine.


 Please wait while we load your content...
Please wait while we load your content...