Enantioselective total synthesis of colomitides and their absolute configuration determination and structural revision†
Abstract
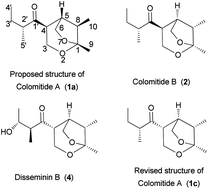
An efficient stereoselective synthetic approach to colomitides, 2,7-dioxabicyclo[3.2.1]octane-type natural products, is reported. Key steps are a stereocontrolled aldol reaction and a gold-catalyzed cycloisomerization. This synthetic strategy has been applied for the first asymmetric total synthesis of the proposed colomitides and their possible diastereomers. Comparison of their 1H and 13C NMR spectra and specific rotations with those of the natural product revealed that the structure of colomitide A should be revised to 1c, and that the absolute stereochemistries of colomitides A and B are 2′R,4R,5R,8S,1R and 2′R,4S,5R,8S,1R.


 Please wait while we load your content...
Please wait while we load your content...