Post-translational modifications involved in the biosynthesis of thiopeptide antibiotics
Abstract
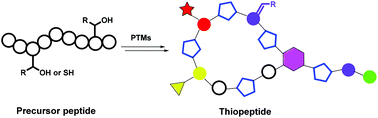
Thiopeptide antibiotics are a class of typical ribosomally synthesized and post-translationally modified peptides (RiPPs) with complex chemical structures that are difficult to construct via chemical synthesis. To date, more than 100 thiopeptides have been discovered, and most of these compounds exhibit remarkable biological activities, such as antibacterial, antitumor and immunosuppressive activities. Therefore, studies of the biosynthesis of thiopeptides can contribute to the development of new drug leads and facilitate the understanding of the complex post-translational modifications (PTMs) of peptides and/or proteins. Since the biosynthetic gene clusters of thiopeptides were first discovered in 2009, several research studies regarding the biochemistry and enzymology of thiopeptide biosyntheses have been reported, indicating that their characteristic framework is constructed via a cascade of common PTMs and that additional specific PTMs diversify the molecules. In this review, we primarily summarize recent advances in understanding the biosynthesis of thiopeptide antibiotics and propose some potential applications based on our insights into the biosynthetic logic and machinery.
- This article is part of the themed collection: Biocatalysis: Natural and biologically inspired synthetic enzymes


 Please wait while we load your content...
Please wait while we load your content...