The application of perfluoroheteroaromatic reagents in the preparation of modified peptide systems†‡
Abstract
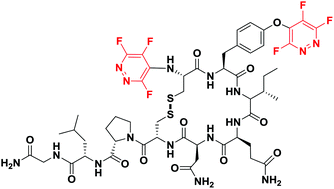
The perfluoroheteroaromatic reagent pentafluoropyridine has proved to be a highly reactive electrophile, undergoing SNAr arylation reactions in the presence of a range of nucleophilic peptide side chains (i.e. cysteine, tyrosine, serine and lysine) under mild conditions. Moreover, we have shown how one-step peptide-modification using perfluoroheteroaromatics can deliver enhanced proteolytic stability in pharmaceutically-relevant peptides such as oxytocin.


 Please wait while we load your content...
Please wait while we load your content...