Synthesis of tri-functionalized MMP2 FRET probes using a chemo-selective and late-stage modification of unprotected peptides†
Abstract
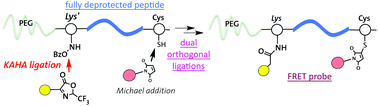
A polymeric FRET probe for the detection of MMP2 was prepared using a new N-hydroxylamine derivative of lysine (1), which was successfully incorporated into the natural peptide sequence by solid phase peptide synthesis (SPPS). Following the attachment of a PEG group to the N-terminus, a peptide was cleaved from the resin. The fully-deprotected peptide–PEG conjugate was subsequently subjected to the α-ketoacid-hydroxylamine (KAHA) ligation and Michael addition of FRET donor (MCA) and acceptor (DNP) moieties, respectively. The successfully synthesized polymeric FRET probes with an MMP2-reactive peptide and a negative control peptide with a random sequence were subjected to an in vitro test with MMP2. This methodology under mild conjugation conditions of KAHA ligation can be applicable for the preparation of NIR probes with sensitive fluorophore moieties.


 Please wait while we load your content...
Please wait while we load your content...