Syntheses and kinetic studies of cyclisation-based self-immolative spacers†
Abstract
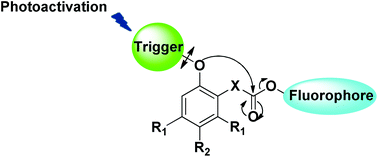
Kinetic analysis of the disassembly of self-immolative spacers based on cyclisation processes was performed. Five compounds were synthesized belonging to two different series, and their kinetic constants were determined. Electron-donating substituents gave a slight acceleration but the main effect was steric, and the Thorpe–Ingold effect was indeed particularly effective. Comparison with the self-immolative spacers based on elimination processes showed that cyclisations gave comparable or lower rate, but the corresponding spacers are more difficult to modulate.


 Please wait while we load your content...
Please wait while we load your content...