Recent advances in organocatalytic enantioselective transfer hydrogenation
Abstract
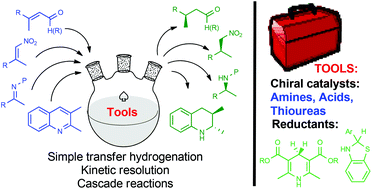
The organocatalytic reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds with biomimetic reductants, e.g. Hantzsch 1,4-dihydropyridine esters and benzothiazolines, is reviewed. Very high yields and stereoselectivities have been achieved with a variety of catalysts, including chiral amines, thioureas and phosphoric acids, even with loadings equivalent to those of transition metal-catalyzed reactions in some cases. Reductive amination reactions and the dearomatization of heteroaromatic substrates are the subject of more than one half of the contributions. Of lately, methodologies based on kinetic resolution, cascade reactions involving transfer hydrogenation and the development of novel reductants have become prominent in an area which brings great prospects for the future of target oriented-synthesis.
N double bonds with biomimetic reductants, e.g. Hantzsch 1,4-dihydropyridine esters and benzothiazolines, is reviewed. Very high yields and stereoselectivities have been achieved with a variety of catalysts, including chiral amines, thioureas and phosphoric acids, even with loadings equivalent to those of transition metal-catalyzed reactions in some cases. Reductive amination reactions and the dearomatization of heteroaromatic substrates are the subject of more than one half of the contributions. Of lately, methodologies based on kinetic resolution, cascade reactions involving transfer hydrogenation and the development of novel reductants have become prominent in an area which brings great prospects for the future of target oriented-synthesis.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...