Oxidation of terminal diols using an oxoammonium salt: a systematic study†
Abstract
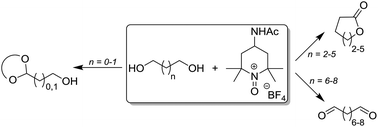
A systematic study of the oxidation of a range of terminal diols is reported, employing the oxoammonium salt 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (4-NHAc-TEMPO+ BF4−) as the oxidant. For substrates bearing a hydrocarbon chain of seven carbon atoms or more, the sole product is the dialdehyde. A series of post-oxidation reactions have been performed showing that the product mixture resulting from the oxidation step can be taken on directly to a subsequent transformation. For diols containing four to six carbon atoms, the lactone product is the major product upon oxidation. In the case of 1,2-ethanediol and 1,3-propanediol, when using a 1 : 0.5 stoichiometric ratio of substrate to oxidant, the corresponding monoaldehyde is formed which reacts rapidly with further diol to yield the acetal product. This is of particular synthetic value given both the difficulty of their preparation using other approaches and also their potential application in further reaction chemistry.


 Please wait while we load your content...
Please wait while we load your content...