Metal-free decarbonylative alkylation–aminoxidation of styrene derivatives with aliphatic aldehydes and N-hydroxyphthalimide†
Abstract
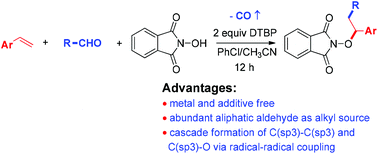
A convenient metal-free decarbonylative alkylation–aminoxidation of styrene derivatives with aliphatic aldehydes and N-hydroxyphthalimide (NHPI) to yield phthalimide protected alkoxyamines is developed. With DTBP as an oxidant and radical-initiator, this reaction smoothly converts aliphatic aldehydes into alkyl radicals and subsequently allows the cascade construction of C(sp3)–C(sp3) and C(sp3)–O bonds via radical–radical coupling.


 Please wait while we load your content...
Please wait while we load your content...