Mimics of pramanicin derived from pyroglutamic acid and their antibacterial activity†
Abstract
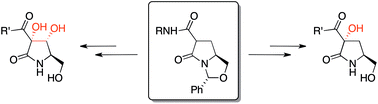
Mono and dihydroxypyrrolidinones are readily available by direct oxygenation of a pyroglutamate-derived bicyclic lactam with high diastereoselectivity, and these may be manipulated further in protected or unprotected form by Grignard addition to a pendant Weinreb amide to give acylhydroxypyrrolidinones, which are analogues of the natural product, pramanicin. Preliminary bioassay against S. aureus and E. coli indicated that some compounds exhibit selective Gram-negative antibacterial activity, and may offer promise for the development of novel systems suitable for antibacterial drug development.


 Please wait while we load your content...
Please wait while we load your content...