Bifunctional bispidine derivatives for copper-64 labelling and positron emission tomography†
Abstract
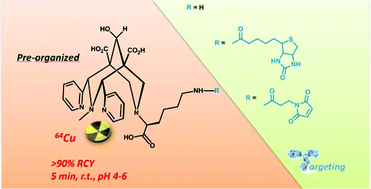
The first radiolabelling studies of a bispidine (3,7-diazabicyclo[3.3.1]nonane) derivative substituted by a glycinate pendant arm (L1) with 64Cu are reported. Labelling was fast and easily performed at room temperature and in a wide range of pH values. Under these conditions, radiochemical yields over 90% were achieved within 5 minutes at micromolar concentration of the ligand. A bifunctional analogue of L1 (L2) has been obtained by introducing an L-lysine amino acid on the bispidine skeleton. Ligand L2 demonstrates good radiolabelling capacities at room temperature and in water (pH 4 to pH 6). This new bispidine is a versatile platform which can easily react with NHS esters and can be subsequently coupled to a recognition unit in order to perform targeted Positron Emission Tomography (PET) imaging. As a proof of concept, two new bifunctional chelators (BFCs) with a biotin (L3) or a maleimide functional group (L4) have been synthesized. The biotinylated BFC is very valuable for pretargeting strategies using streptavidin-conjugated antibodies. The reactivity of the maleimide derivative L4 has been studied with the model peptide GP120. Quantitative coupling has been achieved under physiological conditions, showing a good regioselectivity towards cysteine residues versus lysine amino acids.


 Please wait while we load your content...
Please wait while we load your content...