Conformationally superarmed S-ethyl glycosyl donors as effective building blocks for chemoselective oligosaccharide synthesis in one pot†
Abstract
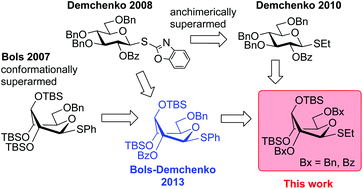
A new series of superarmed glycosyl donors has been investigated. It was demonstrated that the S-ethyl leaving group allows for high reactivity, which is much higher than that of equally equipped S-phenyl glycosyl donors that were previously investigated by our groups. The superarmed S-ethyl glycosyl donors equipped with a 2-O-benzoyl group gave complete β-stereoselectivity. Utility of the new glycosyl donors has been demonstrated in a one-pot one-addition oligosaccharide synthesis with all of the reaction components present from the beginning.


 Please wait while we load your content...
Please wait while we load your content...