Reaction of phosphinylated nitrosoalkenes with electron-rich heterocycles. Electrophilic aromatic substitution vs. cycloaddition†
Abstract
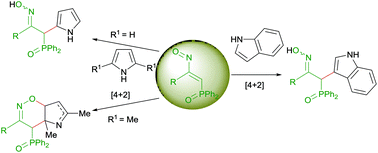
The behavior of phosphinyl nitrosoalkenes with indole, pyrrole and 2,5-dimethylpyrrole is described. The reaction of nitrosoalkenes with indole leads to the formation of 3-substituted indoles. While a concerted asynchronous [4 + 2] cycloaddition process may explain the formation of 3-substituted indole when a methyl group is present at the 3-position of nitrosoalkene, the presence of a 3-methoxycarbonyl group at the same position of nitrosoalkene increases its electrophilic character, and both mechanisms, an electrophilic aromatic substitution and a [4 + 2] cycloaddition process, are predicted to be competitive, although thermodynamically the cycloaddition is favoured. Phosphinyl nitrosoalkenes react with pyrrole leading to the corresponding 2-substituted pyrroles, while the treatment of 2,5-dimethylpyrrole with these nitrosoalkenes gives rise to the formation of bicyclic 1,2-oxazines. The mechanism of the reaction of phosphinyl nitrosoalkenes with pyrrole and 2,5-dimethylppyrrole may be explained by an initial hetero-Diels–Alder cycloaddition in both cases, but only subsequent rearomatization in the case of pyrrole. Theoretical studies show very good agreement with the experimental findings and the proposed mechanisms.


 Please wait while we load your content...
Please wait while we load your content...