A multifunctionalized strategy of indoles to C2-quaternary indolin-3-ones via a TEMPO/Pd-catalyzed cascade process†
Abstract
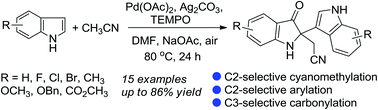
A method for combinative oxidative homo dimerization and cyanomethylation of free indole derivatives catalysed by TEMPO and Pd(OAc)2 was demonstrated for the first time. This new methodology is both atom and step efficient and is applicable to a broad scope of substrates, allowing the synthesis of a range of synthetically valuable 2-(2-(1H-indol-3-yl)-3-oxoindolin-2-yl)acetonitriles in moderate to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...