1,6-Conjugate addition of zinc alkyls to para-quinone methides in a continuous-flow microreactor†
Abstract
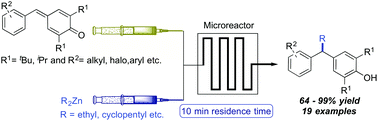
An efficient method for the synthesis of alkyl diarylmethanes through the 1,6-conjugate addition of dialkylzinc reagents to para-quinone methides (p-QMs) has been developed under continuous flow conditions using a microreactor. This protocol allows to access unsymmetrical alkyl diarylmethanes in moderate to excellent yields using a wide range of p-QMs and dialkylzinc reagents. Interestingly, this transformation worked well without the requirement of a catalyst.


 Please wait while we load your content...
Please wait while we load your content...