Asymmetric chemoenzymatic synthesis of 1,3-diols and 2,4-disubstituted aryloxetanes by using whole cell biocatalysts†
Abstract
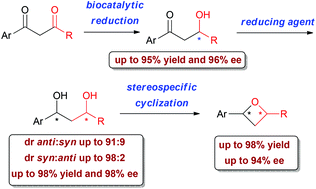
Regio- and stereo-selective reduction of substituted 1,3-aryldiketones, investigated in the presence of different whole cell microorganisms, was found to afford β-hydroxyketones or 1,3-diols in very good yields (up to 95%) and enantiomeric excesses (up to 96%). The enantiomerically enriched aldols, obtained with the opposite stereo-preference by baker's yeast and Lactobacillus reuteri DSM 20016 bioreduction, could then be diastereoselectively transformed into optically active syn- or anti-1,3-diols by a careful choice of the chemical reducing agent (diastereomeric ratio up to 98 : 2). The latter, in turn, were stereospecifically cyclized into the corresponding oxetanes in 43–98% yields and in up to 94% ee, thereby giving a diverse selection of stereo-defined 2,4-disubstituted aryloxetanes.


 Please wait while we load your content...
Please wait while we load your content...