Synthesis of α-functionalized α-indol-3-yl carbonyls through direct SN reactions of indol-3-yl α-acyloins†
Abstract
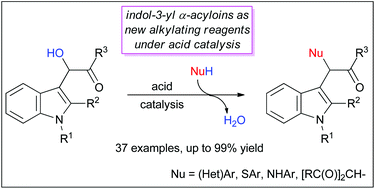
A new and efficient synthesis of α-functionalized α-indol-3-yl ketones from easily available indolyl α-acyloins is reported. This process, catalyzed by Brønsted or Lewis acids, involves an uncommon direct nucleophilic substitution reaction of a secondary α-carbonyl-substituted hydroxyl group. The described methodology allows the introduction of a variety of nucleophiles such as (hetero)arenes, thiophenols, nitroanilines and 1,3-dicarbonyl derivatives. The synthesized α-indol-3-yl carbonyl compounds are important synthetic targets also useful for accessing functionalized tryptophols and furan-3-yl indoles.


 Please wait while we load your content...
Please wait while we load your content...