Regioselective oxidation and metalation of meso-unsubstituted azuliporphyrins†
Abstract
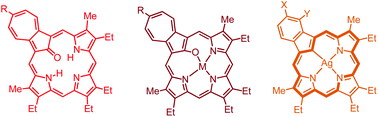
Azuliporphyrins are intriguing porphyrin analogues that incorporate an azulene ring in place of a pyrrolic unit. This system undergoes regioselective oxidation reactions and favors the formation of stable organometallic derivatives. Reaction of meso-unsubstituted azuliporphyrins with Co2(CO)8 or CoCl2·6H2O gave 21-oxyazuliporphyrins, while Cu(OAc)2 produced the corresponding copper(II) complexes. Treatment of an oxyazuliporphyrin with Ni(OAc)2 or Pd(OAc)2 afforded analogous nickel(II) and palladium(II) derivatives. Silver(I) acetate in pyridine reacted with azuliporphyrins to give moderate yields of silver(III) benzocarbaporphyrins, and the prevalence of structures with a formyl moiety at the sterically crowded 21-position suggested that the ring contraction reactions were triggered in part by intramolecular attack from an axial peroxide ligand. Related thiaazuliporphyrins reacted with palladium(II) acetate to give palladium(II) benzothiacarbaporphyrins but this chemistry did not give rise to structures with 21-formyl groups, suggesting that the ring contraction reactions occurred by a different mechanistic pathway. These results demonstrate the existence of a rich tapestry of oxidation and metalation reactions for azuliporphyrin systems.


 Please wait while we load your content...
Please wait while we load your content...