Novel and efficient transformation of enamides into α-acyloxy ketones via an acyl intramolecular migration process†
Abstract
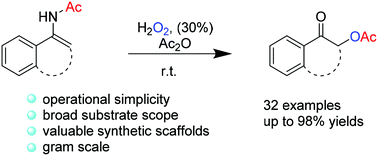
Hydrogen peroxide and anhydride mediated transformation of enamides to afford substituted α-acyloxy ketones is described. This transition-metal-free cascade reaction has a broad substrate scope and high efficiency. The acyl intramolecular migration procedure successfully achieved this acyloxylation process under mild conditions and increased the atom efficiency.


 Please wait while we load your content...
Please wait while we load your content...