Alcohol oxidation with H2O2 catalyzed by a cheap and promptly available imine based iron complex†
Abstract
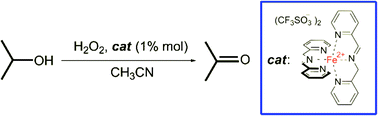
We previously reported that the iminopyridine iron(II) complex 1, easily and quantitatively obtainable in situ, can activate H2O2 to form a powerful oxidant, capable of aliphatic C–H bond hydroxylation. In the present study we expand the application of this catalyst to the oxidation of a series of alcohols to the corresponding carbonyl compounds. The oxidation of aliphatic alcohols proceeds smoothly, while that of benzylic alcohols is shown to be challenging. Some collected pieces of evidence suggest a preference of the oxidizing species for the aromatic ring instead for the alcoholic moiety. The decrease of the electron density in the aromatic ring shifts the oxidation from the aromatic towards the alcoholic moiety. Quite surprisingly, preferential oxidation of cyclohexanol versus benzylic alcohol was achieved, showing unprecedented selectivity.

 Please wait while we load your content...
Please wait while we load your content...