N-Heterocyclic carbene-catalysed intramolecular hydroacylation to form basic nitrogen-containing heterocycles†
Abstract
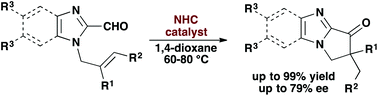
We report catalytic, intramolecular hydroacylations of N-allylimidazole-2-carboxaldehydes and N-allylbenzimidazole-2-carboxaldehydes. These exo-selective hydroacylations occur in the presence of a N-heterocyclic carbene catalyst to generate 5,6-dihydro-7H-pyrrolo[1,2-α]imidazol-7-ones and 1,2-dihydro-3H-benzo[d]pyrrolo[1,2-α]imidazol-2-ones in high yields (66–99%). In addition, hydroacylations of N-allylimidazole-2-carboxaldehydes in the presence of a chiral, non-racemic NHC catalyst occur, forming 5,6-dihydro-7H-pyrrolo[1,2-α]imidazol-7-ones in moderate-to-high yields (39–98%) with modest enantioselectivities (56–79% ee).


 Please wait while we load your content...
Please wait while we load your content...