Computation-guided improved one-pot synthesis of macrocyclic cation-binding aromatic pyridone pentamers†
Abstract
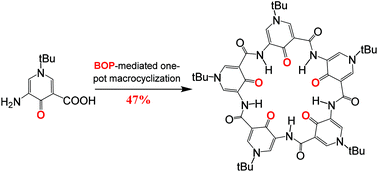
By using polar DMF to relax the H-bonded rigid backbone and to lower the energetic penalty associated with the sterically-crowded environment, the yields for BOP-mediated one-pot synthesis of pentameric macrocycles can be improved from 10–25% as obtained in CH2Cl2 to 13–47% when 15% DMF in CH2Cl2 was used as the reaction medium.

 Please wait while we load your content...
Please wait while we load your content...