Conformational studies of 10–23 DNAzyme in solution through pyrenyl-labeled 2′-deoxyadenosine derivatives†
Abstract
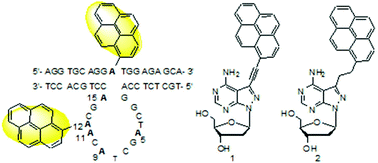
10–23 DNAzyme is a small catalytic DNA molecule. Studies on its conformation in solution are critical for understanding its catalytic mechanism and functional optimization. Based on our previous research, two fluorescent nucleoside analogues 1 and 2 were designed for the introduction of a pyrenyl group at one of the five dA residues in the catalytic core and the unpaired adenosine residue in its full-DNA substrate, respectively. Ten pyrenyl–pyrenyl pairs are formed in the DNAzyme–substrate complexes in solution for sensing the spacial positions of the five dA residues relative to the cleavage site using fluorescence spectra. The position-dependent quenching effect of pyrene emission fluorescence by nucleobases, especially the pyrenyl–pyrenyl interaction, was observed for some positions. The adenine residues in the 3′-part of the catalytic loop seem to be closer to the cleavage site than the adenine residues in the 5′-part, which is consistent with the molecular dynamics simulation result. The catalytic activities and Tm changes also confirmed the effect of the pyrenyl–nucleobase and pyrenyl–pyrenyl pair interactions. Together with functional group mutations, catalytically relevant nucleobases will be identified for understanding the catalytic mechanism of 10–23 DNAzyme.

 Please wait while we load your content...
Please wait while we load your content...