A general approach to iridoids by applying a new Julia olefination and a tandem anion-radical-carbocation crossover reaction†
Abstract
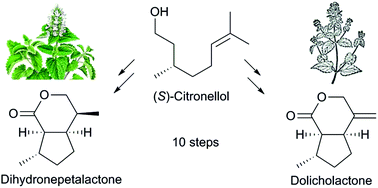
A unified, asymmetric approach to the total synthesis of naturally occurring iridoids is presented. The synthesis features a recently discovered ortho → α transmetalation of alkyl aryl sulfone carbanions, thus enabling Julia reactions, by which so far hardly accessible disilylated olefins have been obtained. A subsequent tandem alkoxycarbonylation/oxidative radical cyclization afforded substituted cyclopentane building blocks with high diastereoselectivity. These compounds serve as unique central intermediates for short access to dihydronepetalactone, dolicholactone and potentially other iridoids.

 Please wait while we load your content...
Please wait while we load your content...