Introduction of a tailor made anion receptor into the side chain of small peptides allows fine-tuning the thermodynamic signature of peptide–DNA binding†
Abstract
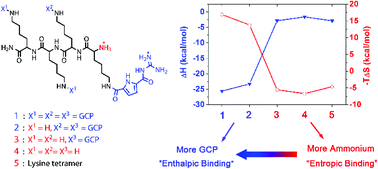
The binding between peptides and DNA is often driven by entropic forces. We demonstrate herein a new approach to shift the thermodynamic profile of peptide/DNA binding from entropy to enthalpy driven. This eventually leads to higher compacted DNA aggregates which are important for gene transfection.

 Please wait while we load your content...
Please wait while we load your content...