Phosphorothioate anti-sense oligonucleotides: the kinetics and mechanism of the generation of the sulfurising agent from phenylacetyl disulfide (PADS)†
Abstract
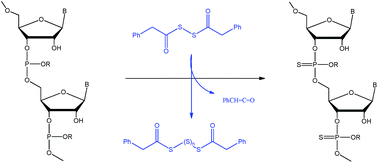
The synthesis of phosphorothioate oligonucleotides is often accomplished in the pharmaceutical industry by the sulfurisation of the nucleotide–phosphite using phenylacetyl disulfide (PADS) which has an optimal combination of properties. This is best achieved by an initial ‘ageing’ of PADS for 48 h in acetonitrile with 3-picoline to generate polysulfides. The initial base-catalysed degradation of PADS occurs by an E1cB-type elimination to generate a ketene and acyldisulfide anion. Proton abstraction to reversibly generate a carbanion is demonstrated by H/D exchange, the rate of which is greatly increased by electron-withdrawing substituents in the aromatic ring of PADS. The ketene can be trapped intramolecularly by an o-allyl group. The disulfide anion generated subsequently attacks unreacted PADS on sulfur to give polysulfides, the active sulfurising agent. The rate of degradation of PADS is decreased by less basic substituted pyridines and is only first order in PADS indicating that the rate-limiting step is formation of the disulfide anion from the carbanion.

 Please wait while we load your content...
Please wait while we load your content...